Abbreviated Electron Configuration For Cobalt
Cobalt is a chemical element with atomic number27 which means there are 27 protons and 27 electrons in the atomic construction. Thechemical symbol for Cobalt isCo.
Electron configuration ofCobaltis[Ar] 3d7 4s2.
Possible oxidation states are+ii,three.
The periodic table is a tabular brandish of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Knowledge of theelectron configuration of different atoms is useful in understanding the structure of the periodic table of elements.
Every solid, liquid, gas, and plasma is equanimous of neutral or ionized atoms. Thechemical backdrop of the atom are adamant past the number of protons, in fact, by number andarrangement of electrons. Theconfiguration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element's electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding beliefs. In the periodic tabular array, the elements are listed in order of increasing atomic number Z.
It is thePauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The ordering of the electrons in the basis country of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there upwards the energy scale until each of the atom's electrons has been assigned a unique set of quantum numbers. This fact has cardinal implications for the building upwardly of the periodic tabular array of elements.
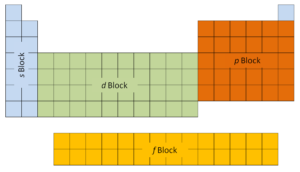 The get-go 2 columns on the left side of the periodic table are where thesouthward subshells are beingness occupied. Because of this, the first ii rows of the periodic table are labeled thes block. Similarly, thep blockare the right-most six columns of the periodic tabular array, thed blockis the middle x columns of the periodic tabular array, while thef cakeis the xiv-column section that is ordinarily depicted as detached from the master body of the periodic table. It could be part of the main body, merely so the periodic table would exist rather long and cumbersome.
The get-go 2 columns on the left side of the periodic table are where thesouthward subshells are beingness occupied. Because of this, the first ii rows of the periodic table are labeled thes block. Similarly, thep blockare the right-most six columns of the periodic tabular array, thed blockis the middle x columns of the periodic tabular array, while thef cakeis the xiv-column section that is ordinarily depicted as detached from the master body of the periodic table. It could be part of the main body, merely so the periodic table would exist rather long and cumbersome.
For atoms with many electrons, this notation can get lengthy then an abbreviated notation is used. The electron configuration can exist visualized as the core electrons, equivalent to thenoble gas of the preceding flow, and the valence electrons (e.g. [Xe] 6s2 for barium).
Oxidation states are typically represented by integers which may exist positive, nil, or negative. Most elements have more than one possible oxidation land. For example, carbon has nine possible integer oxidation states from −4 to +4.
"Oxidation country of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds…"
and the term oxidation number is nigh synonymous. An element that is not combined with any other unlike elements has an oxidation state of 0. Oxidation state 0 occurs for all elements – it is but the element in its elemental form. An atom of an element in a compound will have a positive oxidation state if it has had electrons removed. Similarly, adding electrons results in a negative oxidation state. We have also distinguish betwixt the possible and common oxidation states of every element. For example, silicon has nine possible integer oxidation states from −4 to +iv, but only -iv, 0 and +4 are mutual oxidation states.
32
Ge
Germanium
[Ar] 3d10 4s2 4p2
34
Se
Selenium
[Ar] 3d10 4s2 4p4
51
Sb
Antimony
[Kr] 4d10 5s2 5p3
52
Te
Tellurium
[Kr] 4d10 5s2 5p4
73
Ta
Tantalum
[Xe] 4f14 5d3 6s2
74
W
Tungsten
[Xe] 4f14 5d4 6s2
78
Pt
Platinum
[Xe] 4f14 5d9 6s1
80
Hg
Mercury
[Xe] 4f14 5d10 6s2
104
Rf
Rutherfordium
[Rn] 5f14 6d2 7s2
106
Sg
Seaborgium
[Rn] 5f14 6d4 7s2
109
Mt
Meitnerium
[Rn] 5f14 6d7 7s2
110
Ds
Darmstadtium
[Rn] 5f14 6d8 7s2
111
Rg
Roentgenium
[Rn] 5f14 6d9 7s2
112
Cn
Copernicium
[Rn] 5f14 6d10 7s2
113
Nh
Nihonium
[Rn] 5f14 6d10 7s2 7p1
114
Fl
Flerovium
[Rn] 5f14 6d10 7s2 7p2
115
Mc
Moscovium
[Rn] 5f14 6d10 7s2 7p3
116
Lv
Livermorium
[Rn] 5f14 6d10 7s2 7p4
117
Ts
Tennessine
[Rn] 5f14 6d10 7s2 7p5
118
Og
Oganesson
[Rn] 5f14 6d10 7s2 7p6
64
Gd
Gadolinium
[Xe] 4f7 5d1 6s2
71
Lu
Lutetium
[Xe] 4f14 5d1 6s2
91
Pa
Protactinium
[Rn] 5f2 6d1 7s2
93
Np
Neptunium
[Rn] 5f4 6d1 7s2
103
Lr
Lawrencium
[Rn] 5f14 7s2 7p1
Abbreviated Electron Configuration For Cobalt,
Source: https://www.periodic-table.org/cobalt-configuration-oxidation/
Posted by: demeryarman1949.blogspot.com



0 Response to "Abbreviated Electron Configuration For Cobalt"
Post a Comment